SeriesLock Xgen™ Genderless Quick Coupling for bioprocessing
Description
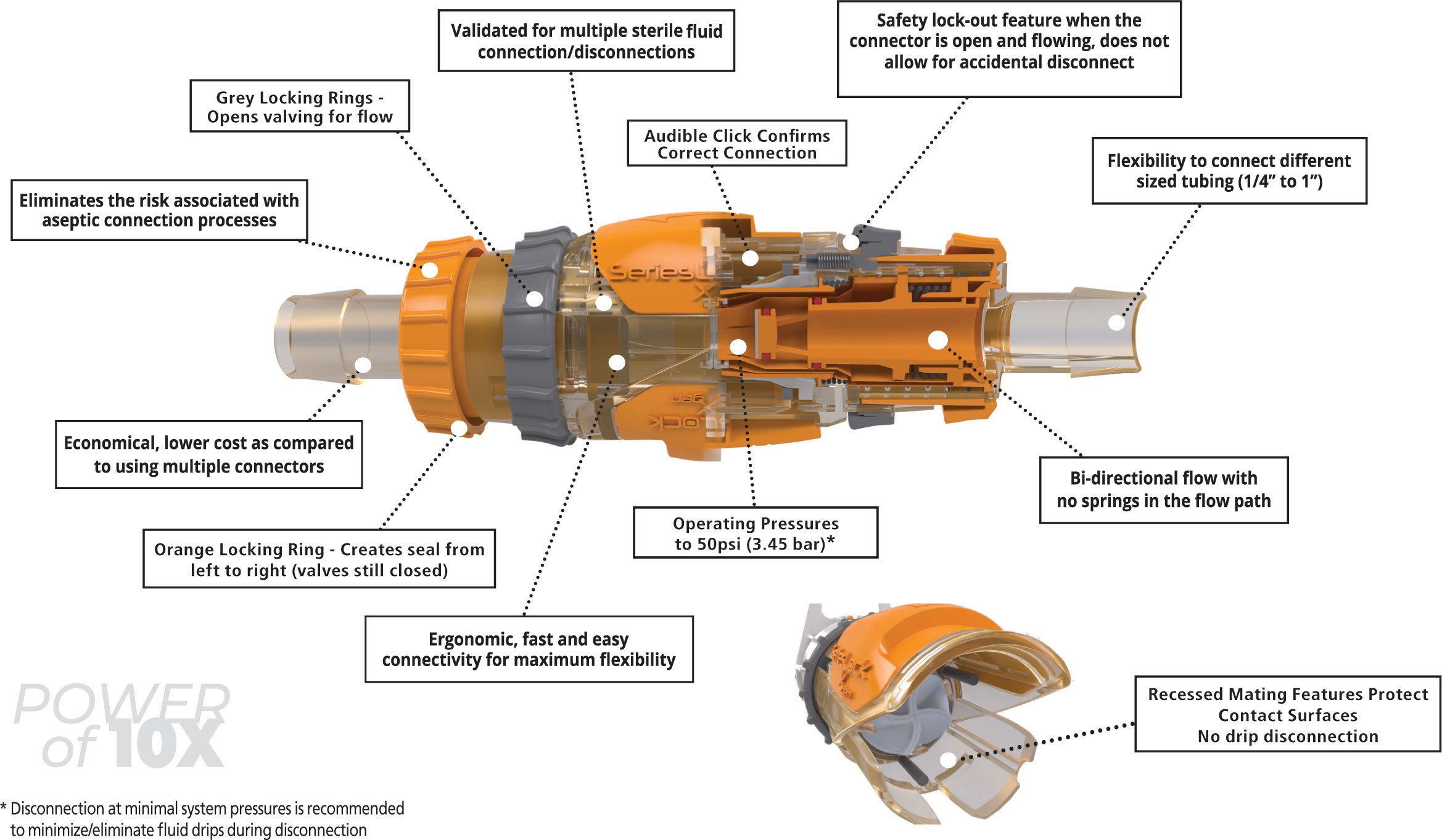
SeriesLockXgen™ is an intuitive, easy to use genderless sterile quick-disconnect solution. Xgen genderless quick connect technology is revolutionizing the bioprocessing industry by offering a more effi cient, versatile, and reliable solution for connecting equipment and processes.
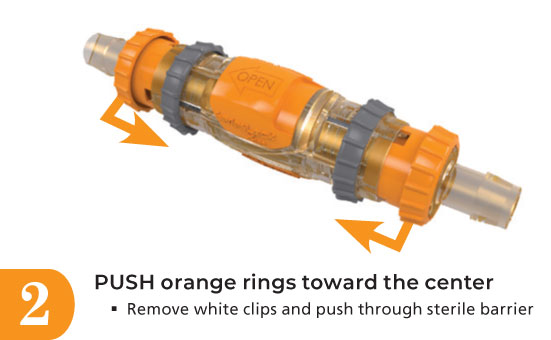
Xgen is revolutionary with a small footprint of 3.6” per half and 5.1” connected. Recessed mating features protect contact surfaces and allows for a sterile, leak free connection, disconnection, and reconnection up to 10 times even in gray spaces.
This genderless connector is manufactured with ISO 10993 compliant materials. All fl uid contacting components are constructed from polysulphone (PSU) and incorporate Class VI silicone seals.
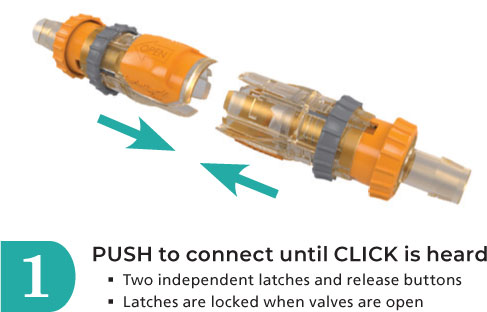
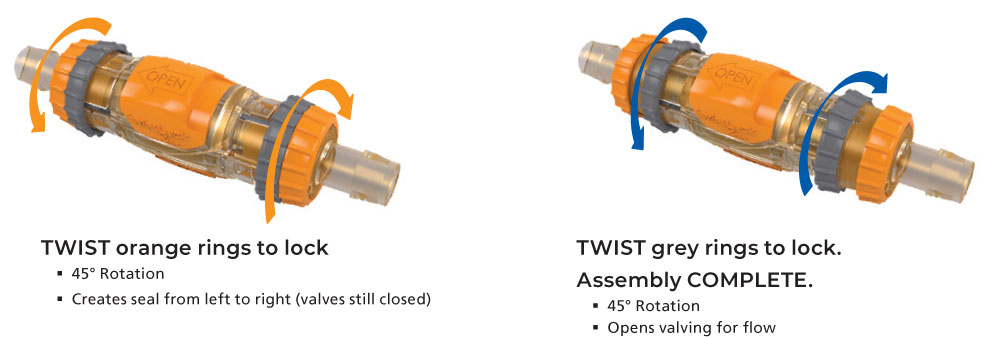
The audible click upon connection, the locking rings and the safety slide release are features to help prevent accidental disconnects. Standard sizes are available to accommodate tubing sizes ranging from 1/4” ID to 1” ID Tubing.
Specifications
Working Pressure
|
3.5 bar
|
Storage Temperature Range
|
-80 °C bis 150°C
|
Service Temperature Range
|
20 °C bis 150°C
|
Sterilization Methods
- Gamma
- E-Beam
- ETO
- Autoclace 121°C (for 45 minutes)
- Autoclave 134°C (for 15 minutes - (Gravity Displacement Method - unwrapped) followed by 15-30 minutes drying time.)
(Typical radiation cycle <50 kGy (end user to verify their use environment and sterilization cycle validations).)
Product Features
- Sterile single-use connection that disconnects and reconnects up to ten times.
- Safety lock-out feature when connector valve is open and fl owing does not allow for an accidental disconnect.
- Reduces inventory with single part numbers in both halves of a symmetrical assembly.
- Audible click confi rms proper connection.
- Bi-directional fl ow.
- Polysulfone housing for toughness and stability at high temperatures.
- Operating Pressures to 3.45 bar
- E-beam, Autoclave and Gamma sterilization stable.
Regulatory
- Superior Bio Compatibility
- Ultra-low Extractables / Leachables
- USP Class VI Compliant
- ISO 10993-4 – Non-hemolytic
- ISO 10993-5 – Non-cytotoxic
- ISO 10933-10– Intracutaneous Reactivity and Irritation / Sensitization
- ISO 10933-11– Systemic Toxicity
- Non-animal derived − BSE/TSE compliant
- Material Certificate and Lot Traceability